The US Food and Drug Administration (FDA) closed out 2025 with 16 hematology/oncology approvals in just six weeks, marking one of the most active periods of the year. Issued between early November and mid-December, the decisions spanned hematologic malignancies and solid tumors moving new evidence into clinical practice.
The approvals covered a wide range of disease settings and mechanisms of action, from CAR T-cell therapy and bispecific antibodies to antibody–drug conjugates, targeted small molecules, and formulation advances. Several actions formalized trends that have been building across major medical meetings, including the continued expansion of immune-based therapies in lymphoid malignancies, growing reliance on molecular selection, and increasing attention to treatment delivery, access, and convenience.
Hematologic cancers were featured prominently in the regulatory surge. New approvals and label expansions extended options in multiple myeloma, marginal zone lymphoma, follicular lymphoma, chronic lymphocytic leukemia, light chain (AL) amyloidosis, and acute myeloid leukemia, reinforcing the FDA’s ongoing shift toward targeted and immune-based approaches across both indolent and aggressive disease. In several cases, accelerated approvals were converted to traditional approval, signaling regulatory confidence in longer-term efficacy and safety data.
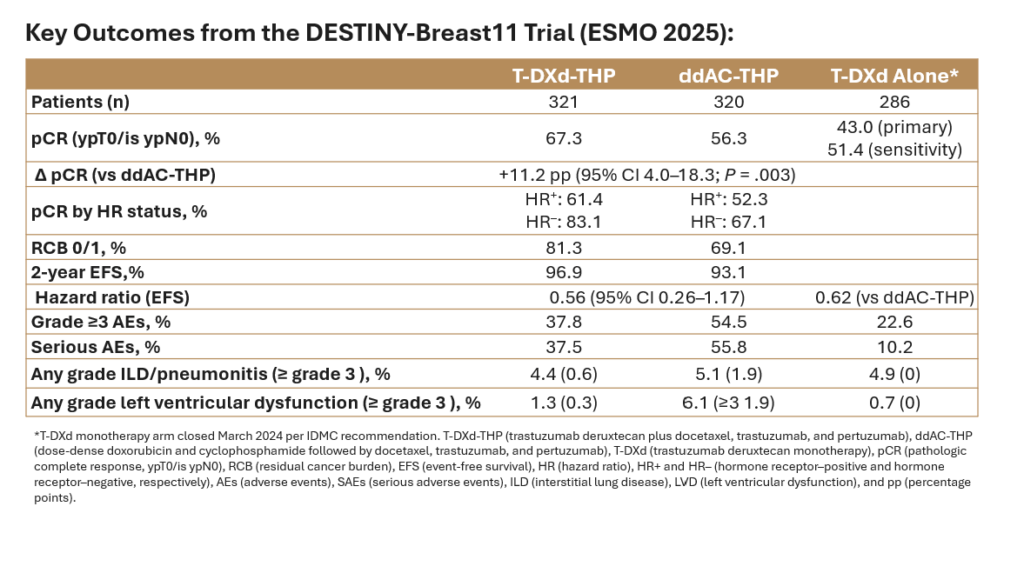
Solid tumor decisions rounded out the period, with notable activity in breast, bladder, lung, gastric, and prostate cancers. These approvals frequently paired established agents with new settings or combinations, underscoring the agency’s emphasis on biomarker-driven treatment and perioperative strategies, particularly in earlier lines of disease.
With these approvals in place, the focus now shifts to ensuring that these advances are accessible and used appropriately in everyday practice.
References:
U.S. Food and Drug Administration. Oncology (Cancer)/Hematologic Malignancies Approval Notifications. FDA website. Updated 2025. Accessed December 2025. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancerhematologic-malignancies-approval-notifications