At the 2025 European Society for Medical Oncology (ESMO) Congress, Nadia Harbeck, MD, of the University of Munich, presented results of the phase 3 DESTINY-Breast11 trial, marking the first randomized evidence that an antibody–drug conjugate–based neoadjuvant regimen can outperform anthracycline-containing chemotherapy in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer (eBC).
The global, open-label phase 3 study randomized 927 patients with HER2-positive eBC to receive (1:1:1) trastuzumab deruxtecan plus docetaxel, trastuzumab, and pertuzumab (T-DXd-THP), dose-dense doxorubicin and cyclophosphamide followed by docetaxel, trastuzumab, and pertuzumab (ddAC-THP), or trastuzumab deruxtecan monotherapy (T-DXd). The primary endpoint of the study was pathologic complete response (pCR) by blinded central review, and key secondary endpoints included event-free survival (EFS), invasive disease-free survival (iDFS), overall survival (OS), and safety.
Median patient age was 50 years, 73% were hormone receptor (HR)–positive, and 89% had node-positive disease.
The trial met its primary endpoint with T-DXd-THP achieving a pCR rate of 67.3% compared with 56.3% with ddAC-THP, an absolute difference of 11.2 percentage points (95% CI 4.0–18.3; P = .003). The benefit was observed in both HR-positive and HR-negative groups and independent of menopausal or HER2 status (IHC3+ vs other) status.
Residual cancer burden (RCB) analyses echoed this finding: 81.3% of patients on T-DXd-THP had RCB 0/1, compared with 69.1% in the control arm.
Event-free survival data were immature at the time of analysis but trended in favor of T-DXd-THP (HR, 0.56; 95% CI, 0.26–1.17). T-DXd monotherapy showed inferior EFS compared to ddAC-THP and was closed on March 13, 2024, as recommended by the independent data monitoring committee.
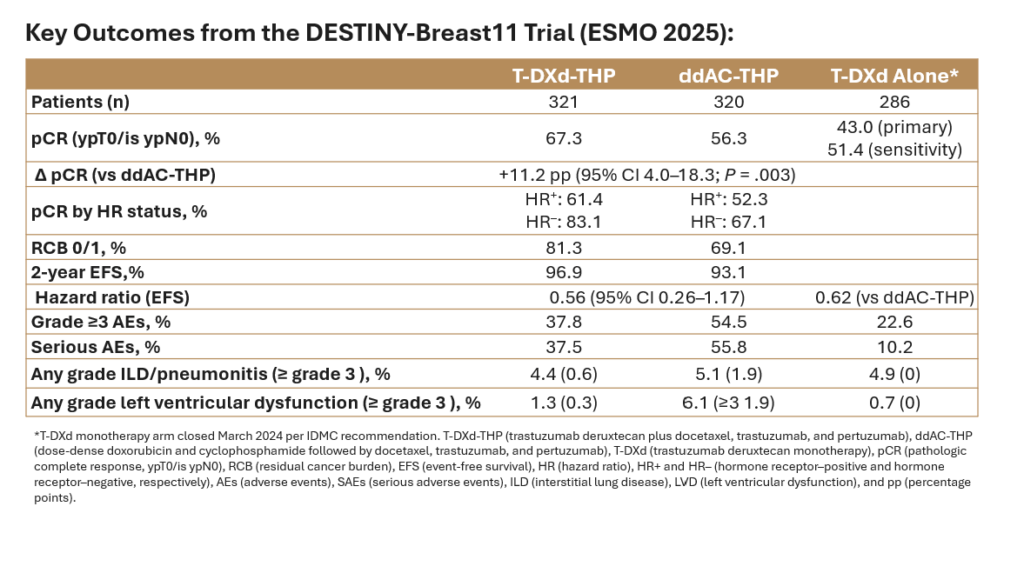
Treatment with T-DXd-containing regimens resulted in fewer grade 3 or higher adverse events (AEs), lower hematologic toxicity, fewer treatment interruptions, and less left-ventricular dysfunction. The incidence of interstitial lung disease (ILD) was comparable between arms. T-DXd-THP treatment resulted in higher rates of nausea but were similar for other gastrointestinal toxicities. Adverse events leading to surgical delay occurred in 3.4% of T-DXd-THP patients vs 2.6% of those receiving ddAC-THP.
Overall, the DESTINY-Breast11study showed that the combination of T-DXd with THP resulted in the highest pCR rates for a registrational trial to date with lower toxicity for patients with HER2+ eBC and may redefine the neoadjuvant standard for high-risk HER2-positive eBC, but longer-term outcomes are awaited. The study was published simultaneously to presentation in the Annals of Oncology.
References
Harbeck N, et al. Trastuzumab deruxtecan–based neoadjuvant therapy in high-risk HER2-positive early breast cancer. Abstract 291O, presented at: ESMO 2025 Congress; October 2025; Barcelona, Spain.
Harbeck N, Modi S, Pusztai L, et al. Neoadjuvant trastuzumab deruxtecan alone or followed by paclitaxel, trastuzumab, and pertuzumab for high-risk HER2-positive early breast cancer (DESTINY-Breast11): a randomised, open-label, multicentre, phase 3 trial.